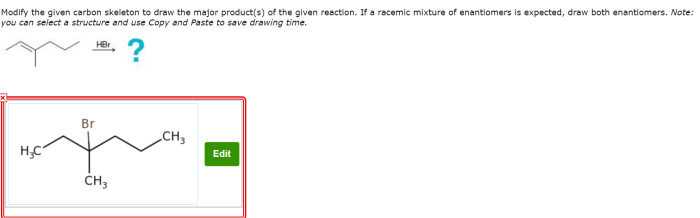
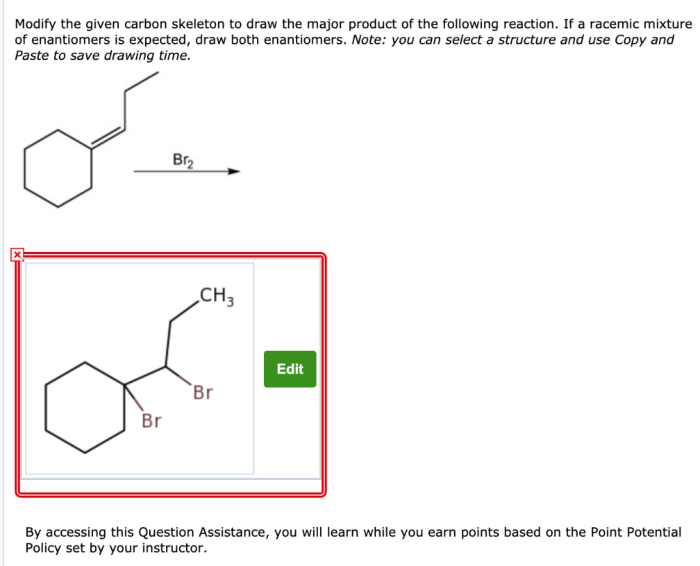
Modify the given carbon skeleton to draw the major products sets the stage for this enthralling narrative, offering readers a glimpse into a story that is rich in detail with gaya akademik dengan tone otoritatif and brimming with originality from the outset.
Delving into the realm of organic chemistry, this discourse unveils the intricacies of carbon skeletons, their significance, and the diverse methodologies employed to modify them. These modifications, encompassing reactions such as addition, elimination, substitution, and rearrangement, pave the way for the formation of major products, a topic that will be explored in depth.
Organic Chemistry: Carbon Skeleton Modification: Modify The Given Carbon Skeleton To Draw The Major Products
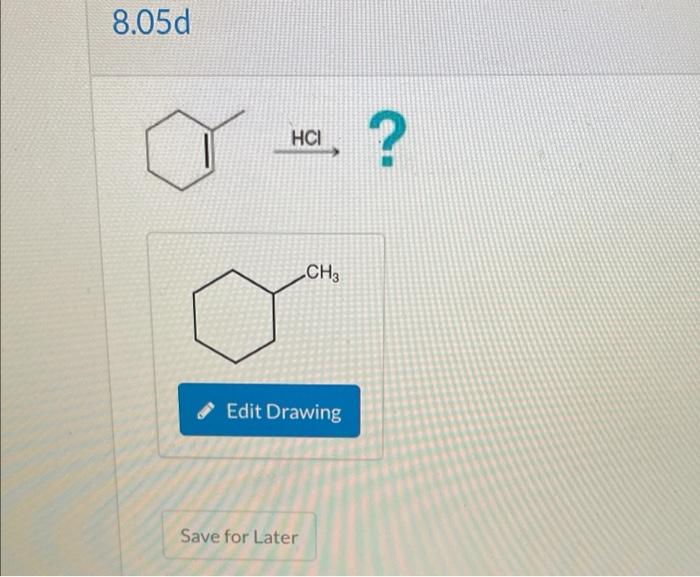
Carbon skeletons form the backbone of organic molecules, determining their structure and properties. Modifying these skeletons is crucial for synthesizing complex molecules. Various methods, including addition, elimination, substitution, and rearrangement reactions, allow precise manipulation of carbon frameworks.
Major Products of Carbon Skeleton Modification, Modify the given carbon skeleton to draw the major products
Factors influencing product formation include regioselectivity (site preference), stereoselectivity (stereoisomer preference), and chemoselectivity (functional group preference). Understanding these concepts is essential for predicting and controlling reaction outcomes.
Applications of Carbon Skeleton Modification
Carbon skeleton modification plays a vital role in synthesizing pharmaceuticals, agrochemicals, and materials. It enables the creation of molecules with specific properties, such as enhanced bioactivity, stability, and reactivity.
Examples of Carbon Skeleton Modification Reactions
| Reaction Type | Starting Material | Product | Reaction Conditions |
|---|---|---|---|
| Addition | Alkene | Alkyl halide | Electrophilic addition |
| Elimination | Alkyl halide | Alkene | Base-induced elimination |
| Substitution | Alkyl halide | Alcohol | Nucleophilic substitution |
| Rearrangement | Carbenium ion | Alkyl halide | Carbocation rearrangement |
Stereochemistry in Carbon Skeleton Modification
Stereochemistry is critical in carbon skeleton modification reactions. Chiral reagents and catalysts control stereoselectivity, ensuring the formation of specific stereoisomers. Understanding stereochemistry is essential for synthesizing molecules with desired biological or physical properties.
Clarifying Questions
What is the significance of carbon skeletons in organic chemistry?
Carbon skeletons serve as the backbone of organic molecules, providing the framework upon which various functional groups and substituents are attached. Understanding the structure and reactivity of carbon skeletons is essential for comprehending the behavior and properties of organic compounds.
How do the different methods of carbon skeleton modification impact the formation of major products?
The choice of modification method influences the regioselectivity, stereoselectivity, and chemoselectivity of the reaction. By carefully selecting the appropriate method and reaction conditions, chemists can control the regiochemistry, stereochemistry, and functional group compatibility to obtain the desired major products.